Our Focus
Tissue repair responses and regeneration are physiological reactions to injuries or inflammation and share common characteristics and phases independently of the injured organ or tissue. For example, upon partial resection of the intestine or liver existing blood vessels are inevitably dissected, which induces acute ischemia (extremely low oxygen levels, O2) and hypercapnia (high carbon dioxide levels, CO2) in the dependent region. Tissue inflammation due to septic conditions can further aggravate this oxygen deprivation, which consequently triggers a protective molecular oxygen-sensing mechanism called the hypoxia-inducible factor (HIF) signaling pathway. Importantly, pharmacologic inhibition of HIF-prolyl hydroxylases (PHDs), the main cellular oxygen-sensors, stimulates the HIF pathway. We, therefore, aim to elucidate the therapeutic potential of PHD inhibitors during tissue repair and regeneration.
Moreover, we investigate the sensing and signaling mechanisms elicited in human cells in response to elevated carbon dioxide. Despite the prevalence of carbon dioxide as one of the most abundant physiologic gases and the primary gaseous product of aerobic respiration, surprisingly little is known about how cells sense, signal, and respond to changes in carbon dioxide. Monocytes and macrophages, which are crucially involved in the inflammatory response and tissue repair, leave the blood stream with normal carbon dioxide levels (5% CO2) and enter sites of inflammation, where carbon dioxide levels are often elevated (10-20% CO2). Because we and others have previously shown a profoundly anti-inflammatory effect of carbon dioxide, we hypothesize that monocytes and macrophages evolved a carbon dioxide-sensing mechanism, controlling immune cell function in the context of tissue repair and regeneration.
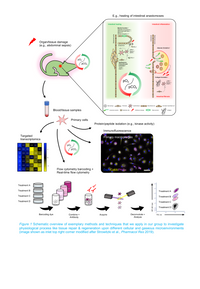
To better understand the role of the gaseous microenvironment during tumor progression, tissue repair, and regeneration, we established novel transgenic animal models and apply state-of-the-art molecular genetic techniques, including CRISPR/Cas9, targeted transcriptomics, and high-throughput flow cytometry (“flow cytometry barcoding”) in our group (Figure 1).
Cell interactions during tumor progression
We investigate cell interaction of cancer-associated fibroblasts or tumor-associated macrophages with colorectal cancer cells. Spatial Omics approaches (e.g., GeoMx) are applied.
The effect of metabolic surgery on hepatic fibrosis
Obese patients suffer from an increased risk for hepatic fibrosis and thus primary liver cancer. In preclinical models and patients samples, we determine the protective effect of metabolic surgery to mitigate fibrosis.
Metabolic signaling during gastric cancer progression
We analyze the expression of enzymes involved in cell metabolism in gastric cancer. Tumor cells are metabolically reprogrammed to decrease proliferation and migration.